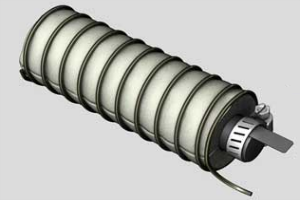
 When going off the grid, it’s safe to assume that most folks will be relying on solar panels for much of their electrical needs. However, a lack of sunlight can present a few problems for any would-be prepper. If only there was a cheap and simple way to supplement a solar panel array on those cloudy days.
When going off the grid, it’s safe to assume that most folks will be relying on solar panels for much of their electrical needs. However, a lack of sunlight can present a few problems for any would-be prepper. If only there was a cheap and simple way to supplement a solar panel array on those cloudy days.
Fortunately, there is such a way, and I’m willing to bet that most of you reading this have never heard of it (I hadn’t until recently). It’s called a Dickens Magnesium Battery after it’s inventor, Stephen Dickens; though the principles behind its function have been around for a very long time. If anything it may be more of a rediscovery, than a completely novel idea.
This device is also called a “Galvanic Cell,” which has been around since the late 1700’s, and possibly even earlier if the theories surrounding the Baghdad Battery are to be believed. It generates small electrical currents by capturing the energy produced by the corrosion of a metal.
In this case, the Dickens battery uses the magnesium as its source of electricity, which many you probably already know if you’ve ever used a fire starter, is a very energy dense material. The design is simple enough that pretty much anyone can make it.
You start out with thick, magnesium rods, which you can buy on Ebay. After that, you’ll need to fasten a metal electrode to the rod with a hose clamp. The metal used for this step is never specified, so feel free to try out a few different metals to see what nets you the best results (more on that in a moment).
After that, you wrap the rod in porous foam, and then coil copper wire around the foam. The idea is to allow water to pass through the foam, but to keep the copper from touching the electrode. Doing so won’t cause anything catastrophic, but your battery will stop producing energy.
After it’s all said and done, it should look like this:
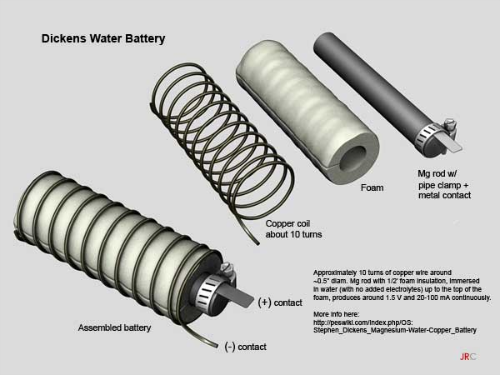
From there, you’ll need a small jar to store this contraption, and you’ll have to puncture holes in the lid to allow the positive and negative contacts to push through. Fill the jar with tap water up to the top of the foam, and close the lid with the contacts exposed. You’ll also need to use something like caulk to seal the holes in the lid, thus keeping the water from evaporating. And that’s it! Your magnesium battery is all done.
But what is it capable of?
Each cell should produce about 1.5 volts, and anywhere from 20mah to 100mah. You’ll notice that the current has a fairly wide range. That’s because this invention hasn’t been around very long, and it’s hard to say what will allow it operate at its optimum efficiency. That’s what I was talking about before with the metal electrode. You’ll have to try a few different metals to see what works best.
Although it doesn’t produce a whole lot of energy, it is pretty cheap, and it will last a really long time. Depending on the current you get from it, it may last more than a year. Maybe even longer. It’s hard to say because to my knowledge, nobody has ever completely depleted the magnesium.
And with 1.5 volts, you can connect 8 of these to produce 12 volts of direct current. Coincidentally, that is exactly what you need if you want to connect it to a deep cycle battery, which are typically used to store the energy produced by solar panels. If you manage to get 8 of these producing 100mah of current, you’ll be pumping a steady stream of 1.2 watts of energy, 24 hours a day, for at least 9 months.
At that point, you’ll have to take the battery apart, and scrape the corrosion buildup off the magnesium and the copper wire. And that’s pretty much the only maintenance you’ll have to do. It’s not a lot of energy, but it adds up after a while, and it’ll be able to supplement a small portion of your energy needs when the sun isn’t out. Or if you don’t mind rapidly depleting your magnesium, you can also add salt to the tap water, which will produce more energy.
For a more detailed description of this device, check out the full instructions for its construction, and hopefully you’ll soon be enjoying your new magnesium battery bank.


Tried this, there is no push behind the voltage (A) so it won’t do much.
2 of them lights a 3v led barely.
It is interesting, I intend to make 4 more and get a bright led going and possibly a tiny electric motor so I have something I can actually see that’s it’s putting power out.
Oh, didn’t have magnesium rod but I do have about 80′ x 4mm of mag ribbon, rolled that around the full length of a pen then doubled it so I used about 6′ for each, I don;t need or expect it to last years but I limited the foam so I can see the magnesium oxidation and degradation in the mason jars.
the link iin the article shows you where you can get reasonably priced magnesium rods.
“…there is no push behind the voltage (A) so it won’t do much.
2 of them lights a 3v led barely. …”
Okay…so you confirmed what the directions state, i.e., “… produce about 1.5 volts, and anywhere from 20mah to 100mah. …” So no surprise there.
most LED’s are specified to operate at 20mA. It would seem that the battery in question was not optimally made.
I tried this d.c current a little different way,using sulfur water and pretty much dirt that stays saturated. The ingredients is simple a steel rod a 1×2 piece of wood using a steel rod a peirce of wood and a thick copper rod = take copper rod wood and steel tie these so the 1×2 is is separated by the wood tie the 3 together tightly and put in a spot that the ground stays really wet and take small copper hook 1 lead to the copper rod and the other to the steel rod ever 2 ft. repeat the longer the lines the more direct current. Do this and to check for success find an old a.c lamp and hook the leads to to the 2 copper leads blow your mind the ground your walking on is your electric current source. Hit me up I am always experiencing. Think about how many generators you’ve thrown away. Every RC toy thrown away has a.c d.c generators that you could have made a ton of free electricity away. I’m just saying!!!
Just be careful when using magnesium products. Magnesiumis a highly flammeable material, and should be kept away from heat sources and open flames.
Well, I am just wondering what a pipe might do. It may if it was a pipe then the electricity might build up bouncing through a pipe and not just off of a rod. Plus maybe putting a bit of small copper wire inside of the the rod might help also.
Any rechargable home made battery possible ?
how about moving the salty water thru a small pump to create water flow which creates electrolosis,just a thought
There is no reason for pump because of convectional flow of ionized molecules.
This won’t work with salted water, it will work the best with deionized water instead or rainwater as natural substitute. Just because salted water will keep energy within, not flow of electrons we need for consumption.
Mr.Harris,
Have you tried it with the salt water flow method? I am excited to hear the results.
What if you shape and form a car battery into the size of a AA battery those care batteries last longer than any other batter being made
Years ago in school we made “Lemon Juice” batteries. Using nails, copper wire and lemon juice (about a 25% mix) while the voltage was lower I remember the teacher saying a core made of silver would work best and give a higher voltage output. Course no one could afford silver rods so…
Silver isn’t expensive. It’s 26$ an ounce it generally runs 13$ But covid has changed that
Very smart reminder. I think small amounts of silver an be acquired through scavenging old mother/ power boards.
Silver quarter?
Reminds me of a earth battery. Could you not use the rod in the center of a large Mason jar, winde a copper wire or ribbon to fit in the inside edge of the Mason jar and be able to achieve a much better voltage using wet dirt or clay as your insulator between positive and negitive???
A good addition to the arsenal of ideas for “free” power.
Interesting. I like the way you think 🤔
how about moving the salty water thru a small pump to create water flow which creates electrolosis,just a thought,an anode would collect all the reaction from the electrolosis which would probably help if was attached to rod,maybe
Has any one tried more wraps of the copper wire? It would be interesting to see what affect that has on the process.