
Since metallic silver is what we want when using colloidal silver as a supplement, when creating the colloid, we want as little ionic silver in the mixture as possible. If you read the previous article on colloidal silver, you should already understand that ionic silver is less desirable as an antimicrobial supplement because of the missing election. Since ionic silver is missing an electron from its outer orbit, it easily bonds to chloride in the body creating silver chloride. The body then expels the compound through urine providing few, if any microbial benefits.
So how exactly is an effective silver colloid made? That’s where things get a little more scientific. While a colloid can have many forms, colloidal silver is one type of colloid that consists of solid particles suspended in a liquid. The solid, in this case, is very small particles (not individual atoms of silver, but clusters of atoms which create particles) of metallic silver and the liquid is water. The “very small particles” in this context refer to particles whose diameter is measured in nanometers. A silver colloid then must have silver particles in suspension. However, Colloidal silver also contains another form of silver called ions. The difference between solutions, colloids, and suspensions is defined by the size of the particles in the liquid.
Since we are focusing on colloidal silver, a colloid contains silver nanoparticles ranging in size from 10-9 m to 10-6 m (1 nm to 1000 nm). A one-nanometer silver particle consists of 31 silver atoms. The diameter of a single silver atom is .288nm.
Colloidal silver is made when an electric current is passed through a series circuit consisting of a silver electrode and de-ionized (DI) water. The current can be either alternating current (AC) or direct current (DC). The current flow causes Ag0 (metal) and Ag+ (ions) to migrate from the electrode into the DI water. AC processes tend to be more efficient than DC in limiting the ionic concentration. It is generally assumed that water ionizes to H + and OH- and that the H +, in the form of the hydronium ion, H3O +, migrates to the cathode, where it is reduced to hydrogen gas, H2, which is liberated. The electrons taken from the cathode are replaced at the anode when Ag metal goes into solution as Ag+.
Therefore, colloidal silver consists of silver in two distinctly different forms, metallic silver particles, and silver ions. The total amount of silver that is reported as the silver concentration (in parts per million) is the sum total of the silver contained in the particles and the silver contained in the silver ions. Typically, silver ions make up 75 to 99 percent of the total silver while only 1 to 25 percent of the total silver is metallic particles.
A solution containing only ionic silver and no particles is not a colloid since there are no solid silver particles in suspension. On the other hand, if 100 percent of the silver was particles and no ions were present, the solution would be a pure colloid. One measure of the quality of a silver colloid is the percentage of silver particles. Ideally, all the silver content would be in the form of particles with no silver ions.
The good news there is it’s almost impossible to get argyria, colloidal silver’s only known side effect, by consuming silver ions. But ionic silver also won’t offer much in the way of antimicrobial benefits either. Accurate measurements of total silver content require the measurement by either atomic absorption or atomic emission of the silver atoms.
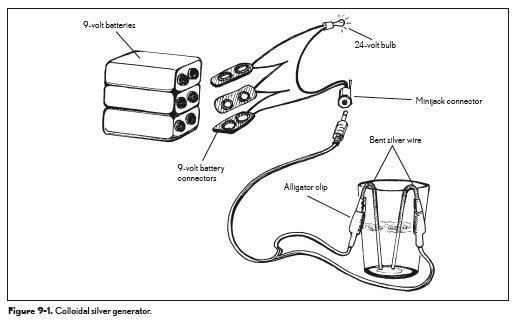
The diagram below shows the setup of a colloidal silver generator. Remember: 9-volt batteries deliver DC. As mentioned previously, to get more metallic silver, AC is more effective. It’s also important to bear in mind that AC batteries are not actually batteries, but converters that create AC current out of DC battery supplies. AC can carry electricity several miles without the loss of power and can also be controlled to increase or decrease power with a transformer. An AC converter on a DC battery creates a more controllable AC energy source with the portability and self-contained benefits of a battery.
Since most manufacturers of colloidal silver do not list the concentration of metallic silver or ionic silver, there’s an observational way to note the difference. When looking at the advertised colloidal silver and the colloidal silver you create at home, you can easily tell by how it looks whether or not there’s a high concentration of ionic silver or metallic silver. Ionic silver solutions should be clear (they look like water) or have a gold tint to them. The particles of ionic silver are too small to be seen except with an electron microscope. When ionic silver is created, bubbles will form on one or both of the silver wires. Colloidal silver is made from silver particles that are microscopic in size. When creating colloidal silver, it will look like wisps of smoke emanating from one of the silver electrodes. The metallic silver particles will usually deliver a gray or silver tint to the solution.
It would be beneficial if colloidal silver manufacturers would list the concentration of ionic silver in their products, but until then, we’ll have to use our observational skills and decide based on appearance.
One of the purest silver collides can be found by clicking here. This particular brand also cites sources and attempts to educate the consumer. You’ll note links to scientific articles below the image of their bottle which will also give you the information we are providing here. MesoSilver also labels the percentage of the metallic silver in their product vs. ionic silver so you’re a well-informed consumer.
In the next colloidal silver article, we will go into detail as to how and why silver particles are more effective than silver ions in the human body.
*This article is for informational purposes only. It is not meant to treat or diagnose.


So I wonder why they show a pool of mercury in the ad!
Colloidal silver is yet another health fraud. Get real people. No scientific evidence that it contributes to health in any way but can be dangerous. Shades of homeopathy nonsense.